Intestinal Mucosa
The term "vitamin A" refers to a category of fat-soluble compounds (retinol, retinal, and retinoic acid)1. To preserve homeostasis, vitamin A and vitamin D regulate the intestinal epithelium and mucosal immune system and shape the gut microbiome2. The intestinal mucosa serves as a buffer between luminal contents and the underlying immune system. The intestinal mucosa has selective permeability due to the physical epithelial barrier, which is closely regulated in homeostasis and impaired during illness. Intestinal permeability is a consequence of a weakened intestinal barrier. The tight junction (ZO; zonula occludens), adherens junction (zonula adherens), and desmosome make up the intercellular epithelial junctions. The epithelium's apical surface forms a single, continuous barrier as a result of the exact alignment of abutting cells. The association of transmembrane claudins, occludins, and ZO proteins to apical cytoskeletal proteins maintain the tight junction as the main regulator of paracellular permeability1.
Vitamin A Effect On Gut Mucosal Immunity
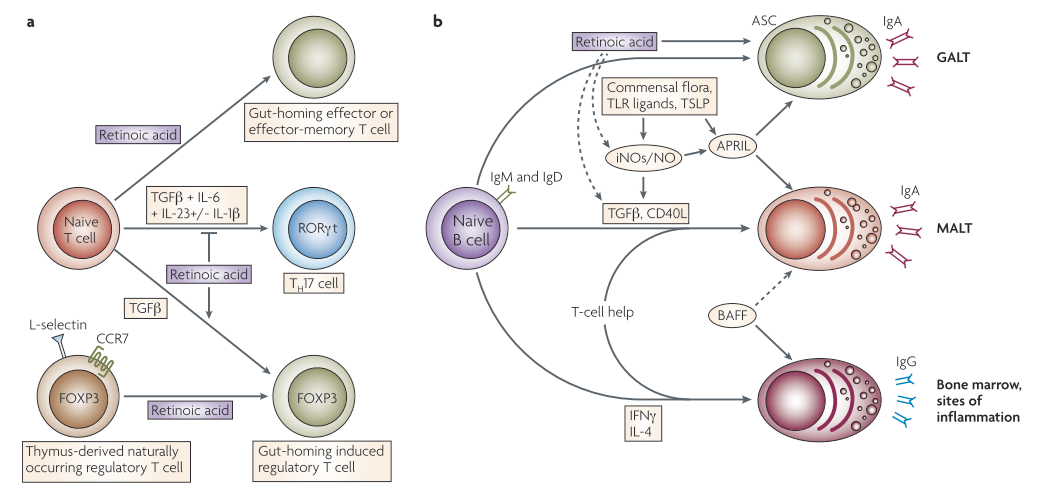
T helper 17 (TH 17) cells are a subclass of CD4+ T helper cells that contain the inflammatory cytokine interleukin 17 (IL17) and are believed to play a role in autoimmune and inflammatory diseases. Regulatory T (TReg) cells are involved in suppressing the effector responses of many other immune cells3. Reduced numbers of intestinal mucosal TRegs have been linked to epithelial tight junction disruption1. Retinoic acid (RA) has been shown to stimulate T-helper-2 (TH2) cell differentiation and upregulate gut-homing receptors' expression. Furthermore, in the presence of transforming growth factor-β (TGFβ), RA inhibits TH 17 cell differentiation and induces forkhead box protein 3 (FOXP3)+ TReg cells by downregulating receptor-related orphan receptor-t (RORt) and inducing FOXP3 expression in T cells. TGFβ-driven activation of TReg cells is also enhanced by RA, which induces gut-homing receptor expression both in naturally occurring and induced TReg cells. TGFβ, interleukin-6 (IL-6), IL-23, and, in humans, IL-1 are all needed for TH17 cell differentiation. B cells stimulated in non-mucosal lymphoid tissues become IgG+ antibody-secreting cells (ASCs), which are present in the bone marrow and inflammatory sites. B cells stimulated in mucosal-associated lymphoid tissues (MALT) produce IgA+ ASCs, on the other hand. TGFβ and CD40 ligand (CD40L) are required for the generation of T-cell-dependent IgA responses in MALT (including GALT), while B-cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) are required for T-cell-independent IgA responses. Toll-like receptor (TLR) signals, commensal bacteria, and thymic stromal lymphopoietin (TSLP) all activate APRIL. TLR signals and commensal bacteria also upregulate inducible nitric oxide synthase (iNOS), releasing nitric oxide (NO), allowing for proper TGFβ signaling, and induces the formation of APRIL and BAFF by dendritic cells. T-cell-dependent and -independent IgA responses both require iNOS and NO. RA may play a direct role in the differentiation of T-cell-independent (and most likely T-cell-dependent) IgA+ ASCs in the GALT. Furthermore, by inducing iNOS expression, RA can indirectly contribute to T-cell-dependent and -independent IgA responses3.
Vitamin A Treatment Options
Despite numerous studies describing vitamin A's beneficial effects on immune response and intestinal epithelial migration and proliferation, little is understood about how vitamin A specifically modulates intestinal barrier function. Tight junction repair can be enabled by RA, which increases ZO-2 expression under inflammatory conditions. In inflammatory conditions, dysbiosis of the microbiota is a central factor in controlling RA effects1. Vitamin A deficiency is linked to compromised intestinal immune responses, which is not surprising, given the importance of retinoic acid in imprinting a gut-homing capability on T and B cells and its ability to facilitate the differentiation of IgA+ ASC. Furthermore, since RA can enhance TGFβ mediated TReg cell activation while inhibiting pro-inflammatory TH 17 cell differentiation, combining RA with TGFβ may be valuable for generating TReg cells for treating inflammatory disorders affecting not only the intestine and also peripheral tissues. RA and retinoic acid receptor (RAR) agonists have been used successfully in autoimmune inflammatory models such as experimental autoimmune encephalomyelitis (EAE), adjuvant arthritis, and experimental nephritis. Psoriasis has been successfully treated with retinoids, and contact dermatitis has been successfully treated and/or prevented in mice and humans3.
Conclusion
Given the important role that vitamin A plays in preserving the intestinal mucosa and its effect on the immune system, evaluating vitamin A status is key in formulating a treatment plan for patients with intestinal permeability dysbiosis. If patients have vitamin A deficiency, adding in foods high in vitamin A such as bell peppers, bok choy, cantaloupe, carrots, dark leafy greens, fish, milk, liver, sweet potato, and tropical fruits would be essential.
References
- de Medeiros PHQS, Pinto D V., de Almeida JZ, et al. Modulation of intestinal immune and barrier functions by vitamin A: Implications for current understanding of malnutrition and enteric infections in children. Nutrients. 2018;10(9):1-14. doi:10.3390/nu10091128
- Cantorna MT, Snyder L, Arora J. Vitamin A and vitamin D regulate the microbial complexity, barrier function and the mucosal immune responses to insure intestinal homeostasis. Crit Rev Biochem Mol Biol. 2019;54(2):184-192. doi:10.1080/10409238.2019.1611734.Vitamin
- Mora JR, Iwata M, Von Andrian UH. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685-698. doi:10.1038/nri2378
*These statements are not meant to diagnose or treat. You should consult your health care provider before starting any new diet, exercise, or supplement.